Chapter 14. Variation of Solubility with Temperature and Solvent
Objectives

- Utilize laboratory techniques to explore two factors that affect solubility: temperature and solute–solvent interactions.
- Quantify the solubility and use graphing techniques to study the solubility trends as a function of temperature.
- Compare the presentation of solubility data using different units.
Introduction
Solubility is an important topic in chemistry. It has been introduced previously, here we come back to the idea, and later in this course we will talk about it again. Solubility is fundamental to many life processes and a solid understanding is necessary for scientists, health professionals, and engineers alike. Solubility is a physical property that may be quantified. Through daily experiences we know that some things can become more or less soluble if we change the surrounding conditions. If you like to sweeten your tea you know that sugar will dissolve much more easily in hot tea than it will in iced tea. In this lab we will consider some factors that affect solubility.
Connection to Lecture
We began the discussion of solubility in our previous chemistry course with the table of solubility rules. This table summarizes the rules for solubility and allows us to classify compounds as soluble or insoluble in water. In lecture now we are learning that we can quantify the solubility of solutes in solvents; two compounds may be soluble in the same solvent, but likely one compound is more soluble than the other. We are learning that different factors can influence how soluble something is. Factors that affect the solubility include
- temperature effects
- solute–solvent interactions
- in gases, pressure effects
In this lab you will be looking at temperature effects (Parts A and B) and solute–solvent interactions (Part C) on the solubility of a compound.
Temperature Effects
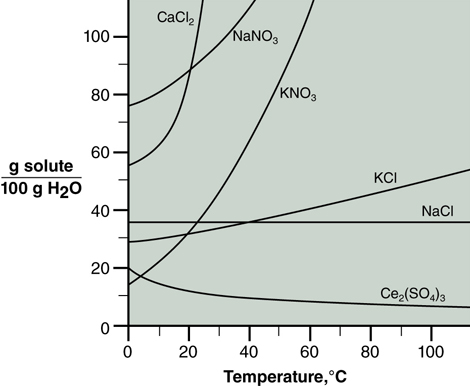
It is no surprise that solubility depends on temperature. The relationship for several substances is shown graphically in Figure 14.1. As illustrated, solubility of an ionic solute in water generally increases with increasing temperature, although there are exceptions. Note two other things about the data displayed. First, each substance has a unique curve, and secondly, dependence of solubility on temperature is generally not a linear relationship. Solubility is an experimentally determined property.
Explanation of the effect of temperature on solubility is in terms of competing processes and energies. Consider formation of an aqueous solution of potassium iodide. Before the solution can form, the solid KI must be pulled apart—forces of attraction between K+ ions and I– ions must be overcome. This process requires energy. The separated ions are then surrounded by layers of water molecules—they become hydrated. This process evolves energy because of the electrostatic attractions between the charged ions and the polar water molecules.
Question 14.1: Is this process endothermic or exothermic? What is the sign of ΔH?
A much smaller amount of energy is involved in separating water molecules from each other and it is included in the hydration energy. The process of solution can be summarized using an energy diagram, Figure 14.2.
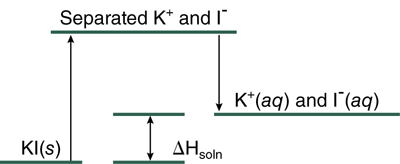
The overall energy change for the process of solution, ΔHsoln, is the difference between the energy of the solution K+(aq) and I–(aq) and the energy of the solute KI(s) to be dissolved. In this case, ΔHsoln is positive and energy is required when KI dissolves in water. This can be indicated by writing heat as a reactant in the equation for the solution process.
KI(s) + water + heat → solution
If we increase the temperature by adding heat, we are supplying a reactant so more solution should form, provided both KI and water are available.
Question 14.2: Does the solubility of KI increase or decrease with increasing temperature?
The strength of attraction between the ions in the solid and the strength of hydration both depend on the size and charge of the ions. If the energy of hydration is greater than the energy required to pull the solid apart, the overall heat of the solution will be negative (ΔHsoln < 0) and heat will be evolved when the solution forms.
Solid + water ↔ solution + heat
In this case, increasing the temperature supplies a product, which is a reactant for the reverse reaction. Increasing the temperature causes the reverse reaction; solid crystallizes out of solution and solubility decreases. This is the case for Ce2(SO4)3 in Figure 14.1.
Solute–Solvent Interactions
Solubility also depends on the nature of the solute and the solvent. The fact that each substance has a unique curve suggests that solubility depends on the nature of the solute. Similarly, if you use the same solute and change the solvent, you would also expect unique behavior. A generalization can be made about solubility and the nature of solute and solvent: Substances with similar intermolecular forces of attraction tend to be soluble in each other. This is stated concisely as “like dissolves like.”
For a solution to form, intermolecular attractions between particles in the solute must be overcome, attractions of solvent particles for each other must be overcome, and new attractions must exist between solute and solvent. In other words, solute–solvent attractions must be strong enough to compete with solute–solute and solvent–solvent attractions.
Consider again the process of dissolving potassium iodide in water. Attractions between particles in the solute are electrostatic attractions between positive ions and negative ions, K+ and I−. Attractions between solvent particles (H2O molecules) are hydrogen bonding, dipole–dipole, and London dispersion forces. The ion–dipole forces of attraction between K+ ions and the negative end of a water molecule, and between I− and the positive end of a water molecule are strong enough to compete with the solute–solute and solvent–solvent attractions. The solution forms. The solubility of KI is 128 g/(100 g H2O) at 0 °C.
If we try to dissolve KI in a nonpolar solvent such as CCl4, the weak London forces of attraction between ions and CCl4 molecules cannot compete with the ion–ion forces in KI. Potassium iodide is essentially insoluble in CCl4.
Now consider dissolving I2 in water and also in CCl4. London forces hold the solid I2 together. The only attraction between the solute and solvent is the London force. Water has very weak London forces; carbon tetrachloride has relatively strong London forces. The solubility of I2 is 100 times greater in CCl4 than in water. Again, “like dissolves like.”
Data Analysis and This Lab
Recall from class that a saturated solution is a solution in which both dissolved and undissolved solute are in equilibrium with each other. That is to say, a saturated solution contains as much solute as it can hold while in equilibrium with excess solid solute. We will make use of the saturation, the point at which no further solute can dissolve in solution, to quantify the solubility of our compound. Solutes will be dissolved in heated solutions and then allowed to cool. When the temperature is such that solute begins to crystallize, the saturation equilibrium is established. In your measurements you will be finding the temperature at which the first crystal forms. Known amounts of solute and solvent are heated and stirred until all of the solute dissolves. The solution is stirred while it cools, and watched closely until the first crystals form. The temperature is recorded. Additional solvent is added to the same test tube and the heating and cooling processes are repeated. The temperature is recorded at each concentration. This is the general procedure for each part of the experiment.
You will use the same solute for all parts of the experiment; it will be either potassium dichromate, K2Cr2O7, an orange, inorganic salt or oxalic acid dihydrate,H2C2O4 • 2 H2O, a white organic acid. For Parts A and B, the solvent is water. The solvent for Part C is a water/dioxane mixture. Dioxane is a nonpolar molecule. It is soluble in water because the oxygen atoms in the molecule have unshared pairs of electrons that are used to form hydrogen bonds to water molecules.
When studying solubility it helps to quantify how much solute is able to dissolve in a given solvent. Solubility is the amount of substance that will dissolve in a given amount of solvent or solution. Common units of solubility are g solute/(100 g solvent). Another concentration unit that we will use for solubility in this experiment is mole fraction, X.
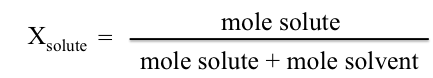
For each solution, determine grams of solute in 100 g solvent using dimensional analysis. For aqueous solutions, assume a density of water of 0.9975 g/mL at 23 °C. The density of the water/dioxane solvent is 1.023 g/mL.
Construct a graph using software (e.g., Excel) with solubility on the y-axis and temperature on the x-axis. While the graph and points should be plotted on the computer, the smooth curve may be drawn in by hand once the graph is printed. Data for aqueous solutions (Parts A and B) should fall along a smooth curve. Data for the mixed solvent (Part C) should form another curve. Plot both sets of data on the same graph and draw the curves in by hand.
Different substances in the same system are often related in molar amounts, whether they are reacting or simply present as a mixture. For each of the aqueous solutions, calculate the mole fraction of the solute.
For water as the solvent (Parts A and B), construct a second graph of temperature on the y-axis versus mole fraction on the x-axis. The data should fall along one smooth curve.
Materials Required
Equipment
- 10-mL and 5-mL pipets
- hot water bath (600-mL beaker)
- cold water bath (400-mL beaker)
- Bunsen burner, wire gauze
- thin-walled rubber tubing
- ring stand, ring
- solubility apparatus
Chemicals
- oxalic acid dihydrate, H2C2O4 • 2 H2O
- potassium dichromate, K2Cr2O7
- 70:30 water:dioxane solution, H2O:C4H8O2
- ice
Common Equipment
- analytical balance
Cautions
Dioxane is a suspect carcinogen. The vapor might be irritating to lungs and mucous membranes. The student hoods should be on. Potassium dichromate is corrosive. Oxalic acid is poisonous. If any of these substances come into contact with your skin or clothing, flood the affected area with water. Be careful to avoid burns from the ring, beaker, and open flame. Goggles must be worn at all times.
Procedure
Answer questions in your lab notebook as you go along. Discussions with your peers and TA are encouraged.
- Obtain the solubility apparatus from your lab instructor. It consists of a thermometer in a rubber stopper in a 75-mL test tube. The coiled end of the wire stirrer should encircle the thermometer, as shown below.
- Your lab instructor will assign oxalic acid or potassium dichromate for use in the experiment.
- Dry the inside of the test tube with a Kimwipe. Weigh your assigned solid, between 6.3 to 6.7 g of oxalic acid or 5.8 to 6.2 g of potassium dichromate, on the analytical balance and add it to the test tube.
- Use a pipet to deliver 10.00 mL of distilled water to the test tube.
- Dissolve the sample completely by heating in hot water. Periodically take the test tube out of the beaker and swirl or shake the test tube, and stir the solution with the wire stirrer, to speed the process. When you stir the solution with the wire stirrer, make sure the coil encircles the thermometer and use slow up-and-down movements. Rapid stirring can cause the thermometer or the test tube to break.
- After the solid is completely dissolved, remove the test tube from the water bath and allow the solution to cool, while gently stirring, to maintain a uniform temperature and concentration. Record the temperature at which crystals are first observed.
- Use a pipet to deliver an additional 5.00 mL of distilled water to the test tube. Repeat steps 5 and 6.
- Repeat step 7 two more times for a total of four determinations with different volumes. Be careful to stir all of the solution, especially when a large volume is used.
- Collect the solution in a waste beaker.
- Clean and dry the inside of the test tube. Weigh between 1.7 to 1.9 g of your assigned solid on the analytical balance and add it to the test tube.
- Use a pipet to deliver 10.00 mL of distilled water to the test tube.
- Dissolve the sample completely by heating in hot water. Periodically take the test tube out of the beaker and swirl or shake the test tube, and stir the solution with the wire stirrer, to speed the process. When you stir the solution with the wire stirrer, make sure the coil encircles the thermometer and use slow up-and-down movements. Rapid stirring can cause the thermometer or the test tube to break.
- After the solid is completely dissolved, allow the solution to cool, while gently stirring, to maintain a uniform temperature and concentration. Record the temperature at which crystals are first observed. It may be necessary to cool the tube in an ice bath at low concentrations.
- Use a pipet to deliver an additional 5.00 mL of distilled water to the test tube. Repeat steps 12 and 13.
- Repeat step 14 one more time for a total of three determinations. Collect the solutions in a waste beaker.
- Clean and dry the inside of the test tube. Weigh your assigned solid, between 6.5 to 7.0 g of oxalic acid or 2.8 to 3.2 g of potassium dichromate, on the analytical balance and add it to the test tube.
- Use a pipet to deliver 10.00 mL of 70:30 water:dioxane mixture to the test tube.
- Dissolve the sample completely by heating in hot water. Periodically take the test tube out of the beaker and swirl or shake the test tube, and stir the solution with the wire stirrer, to speed the process. When you stir the solution with the wire stirrer, make sure the coil encircles the thermometer and use slow up-and-down movements. Rapid stirring can cause the thermometer or the test tube to break.
- After the solid is completely dissolved, allow the solution to cool, while gently stirring, to maintain a uniform temperature and concentration. Record the temperature at which crystals are first observed.
- Use a pipet to deliver an additional 10.00 mL of 70:30 water:dioxane mixture to the test tube. Repeat step 18 and 19.
- Repeat step 20 two more times for a total of four determinations. Collect the solutions in a waste beaker and clean and dry the test tube.
Waste Disposal
All solutions containing potassium dichromate will be orange and must be collected in the inorganic salts beaker. All solutions containing oxalic acid will be colorless (may contain white solid) and must be collected in the organic solvents beaker. Fill in the corresponding waste disposal sheet. Your lab instructor will dispose of the total volumes in the appropriate containers.
Points to Consider
In this lab you are studying the solubility trends with temperature and solvent.
- Include your report sheet with sample calculations and printouts of the two graphs made, with the curves drawn in by hand.
- What do your graphs show, with respect to the solubility trends as a function of temperature?
- Is the trend that you see what was expected based on the material you have read about in the text and learned in lecture?
- Do all data points fit this trend? Are there any known errors to mention for this experiment?
- What do you notice about the solubility in water versus the water:dioxane solvent?